
생화학분자생물학회입니다.
신경세포 내 소기관들의 칼슘 조절 메카니즘 및 기능에 대한 연구
작성자
관리자작성일자
2019-11-11조회수
859신경세포 내 소기관들의 칼슘 조절 메카니즘 및 기능에 대한 연구

권석규
KIST(뇌과학연구소)
skkwon@kist.re.kr
02-958-7217
서론
우리 몸의 기능이 심장, 간, 뇌와 같은 다양한 기관(organ)들에 의해 조절이 되듯이 각각의 세포들도 여러가지 세포내 소기관(intracellular organelle)들에 의해 기능이 조율된다. 신경세포에도 이러한 세포내 소기관들이 존재하고 있으며, 특히 이들 중 에너지 생산, 즉 ATP 생산에 관여한다 알려진 mitochondria(마이토콘드리아)와 단백질 합성 등에 관여하는 endoplasmic reticulum(ER, 소포체)이 큰 볼륨을 차지하고 있다. 이들 소기관은 세포내 칼슘을 흡수하거나 내보냄으로써 농도를 조절할 수 있는데 이러한 칼슘 농도는 신경세포 기능에 굉장히 중요한 기능을 하고 있다. 이에 더해 ER과 mitochondria는 서로 구조적으로 연결되어 mitochondria-ER contacts(MERCs) 혹은 mitochondria-associated ER membrane(MAM)이라 불리우는 구조를 형성하는데 이는 지질(lipid) 합성, mitochondria 모양 조절 및 칼슘 이동 등에 관여한다고 알려져 있다(1-3). 이러한 연결은 여러 신경퇴행성 질환에도 연관되어 있음이 지속적으로 보고되고 있지만 아직 신경세포에서 이들 소기관의 칼슘 조절이 시냅스 기능에 미치는 영향에 대한 연구는 기술적인 한계로 많이 진행되지 않아 왔다.
본론
하나의 신경세포에서 부위별로 특이적인 mitochondria의 모양
신경세포는 신호를 주는 axon(축삭돌기)와 신호를 받는 dendrite(수상돌기)가 cell body(세포체)로부터 가지 모양으로 뻗어나온 독특한 polarized structure를 가지고 있다. 이에 더해 세포내 소기관 또한 axon과 dendrite에서 완전히 다른 모양을 보여준다 (그림 1). Axon에서의 mitochondria는 작고 둥근 모양을 하고 있으며, dendrite에서는 긴 튜브모양을 하고 있다. 이러한 mitochondria의 모양은 fusion과 fission에 의해서 조절될 수 있는데, 알츠하이머나 파킨슨 등 퇴행성 신경질환에서 mitochondria가 작게 쪼개진 모양들로 존재하는 것이 관찰되어 왔다(4-6). 하지만 이들 모양이 정상적인 조건에서 어떻게 유지되는지에 대한 연구는 부족했었다.
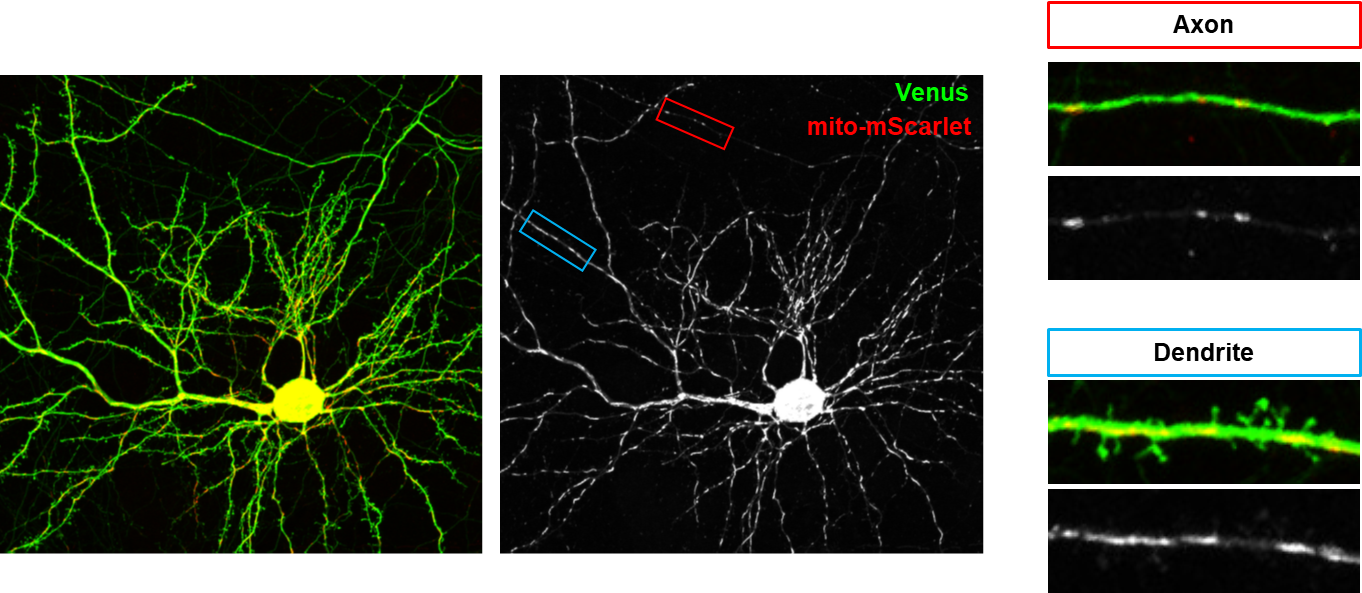
그림 1. Axon과 Dendrite의 mitochondria 모양
Dendrite에서 길고 axon에선 작은 mitochondria 모양에 근거하여 dendrite에서는 mitochondrial fusion이 axon에서는 mitochondrial fission이 dominant하다는 가설에 대한 연구가 최근 진행되었다. Mitochondrial fission과 관련한 단백질 중 신경세포에 많은 MFF를 타겟으로 knockdown이 수행됐을 때, 놀랍게도 dendrite의 mitochondria 모양은 변화가 없었지만 axonal mitochondria가 길어진 것이 관찰되었다(그림 2A-B)(7). 이러한 모양 변화는 presynapse(전시냅스)에 다양한 기능적 변화를 가져왔다.
Axonal presynapse(전시냅스)에서 mitochondria의 역할 연구
기존 연구들에서 뇌에 존재하는 많은 신경세포들이 하나의 axon에 여러 개의 presynaptic release site를 갖고 있는 것이 알려졌으며, 흥미롭게도 50% 이하의 presynaptic site만 mitochondria와 겹쳐있는 것이 관찰되었다. Presynapse에서 action potential에 따른 Ca2+ influx가 presynaptic vesicle exocytosis를 유도하게 되는데 위와 같은 mitochondria의 존재 유무가 Ca2+ clearance를 통해 release 차이를 줄 수 있는지는 밝혀져 있지 않았었다. 헌데 각 presynapse의 release를 이미징 기술을 통해 살펴보았을 때, 놀랍게도 mitochondria가 없는 곳에서 presynaptic Ca2+과 release가 더 올라가 있음이 보여졌다(8).
Mff knockdown neuron에서는 길어진 presynaptic mitochondria가 Ca2+의 over-uptake를 유도하고 이로 인해 presynaptic release가 줄어드는 것이 관찰되었다(그림 2C). 이는 synaptic activity에 큰 영향을 받는 axon branching을 현저히 줄어들게 하는 결과를 가져왔다(그림 2D). 따라서 presynaptic mitochondria에 의한 Ca2+ 조절이 synaptic release property 및 axon branching development에 굉장히 중요하며, presynapse의 mitochondrial size를 작게 유지하는 것이 정상적인 presynaptic function에 필수적임이 규명되었다(7).
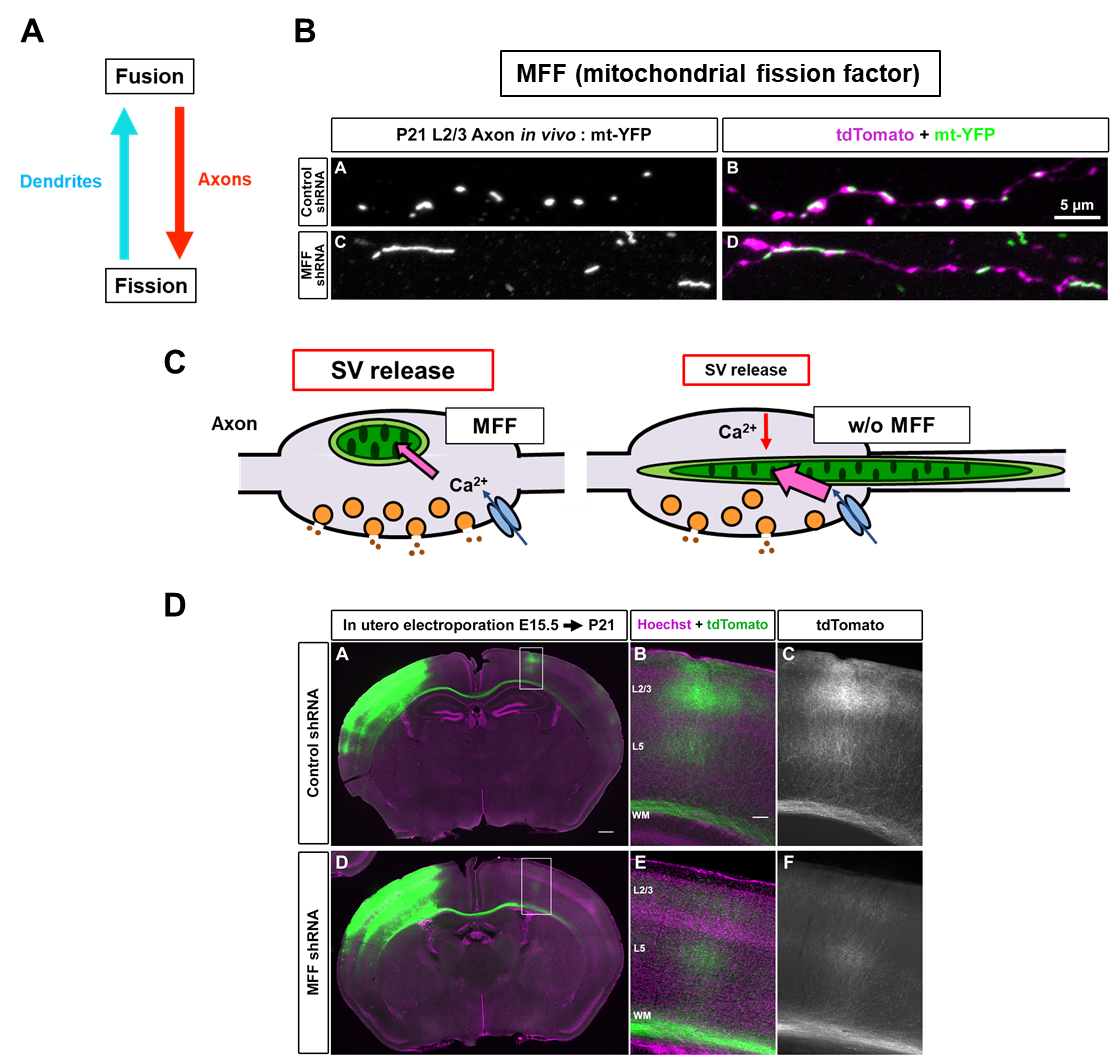
그림 2. MFF에 의한 axonal mitochondria 모양 및 기능 조절.
(A) Axon에서는 mitochondrial fission이 dendrite에서는 mitochondrial fusion이 dominant 하다는 가설을 세우고
mitochondria fission을 조절하는 MFF를 통해 기능을 살펴봄. (B-D) Mff-deficient neuron에서 axonal mitochondria의 elongation이 일어나고
이로 인해 presynaptic Ca2+이 over-uptake되어 neurotransmitter release가 줄어들고 axon branching defect가 나타남.
Dendrite(수상돌기)에서 ER과 mitochondria의 역할 규명
앞서 언급한 바와 같이 비신경세포에서 mitochondria-ER contact의 다양한 기능들이 보고 되어왔다. Mitochondria와 ER은 모두 Ca2+을 흡수하고 방출하는 기능을 하는데 mitochondrial calcium uniporter (MCU)의 경우 Ca2+ affinity가 낮아 ER에서 방출된 Ca2+이 ER-mitochondria 연결을 통해 전달이 될 때 채널이 열리면서 mitochondria로의 흡수가 이루어질 수 있다는 보고들이 있었다(9, 10). 하지만 이것의 직접적인 관찰은 최근에 들어서야 새로운 sensor 개발로 가능하게 되었다. 살아있는 세포내의 Ca2+ dynamics를 보기 위해 다양한 chemical 혹은 genetic Ca2+ sensor들이 개발되었고, 특히 적은 양의 Ca2+에 대한 민감도(sensitivity)를 올리기 위해 이들의 Ca2+ affinity는 높아지는 방향으로 진행되었지만 ER의 resting Ca2+ 농도의 경우 1mM에 이를 정도로 높아 기존의 Ca2+ sensor로 측정하기에는 saturation으로 인한 한계가 있었다. 따라서 최근 ER의 Ca2+ dynamics를 측정하기 위해 affinity가 낮은 ER-targeted genetically-encoded Ca2+ sensor들이 개발되었다(11, 12).
신경세포에서 Ca2+은 시냅스의 기능 조절, 전사 조절 등에 다양한 역할을 하고 있으며, ER에서의 Ca2+ 방출이 시냅스 자극시에 일어난다는 보고들은 있었으나 mitochondria와의 상호작용에 대해서는 알려져 있지 않았다(13-17). 최근 연구에서 신경세포의 ER과 mitochondria Ca2+ dynamics를 새로이 개발된 다른 파장의 형광을 내는 genetically-encoded Ca2+ sensor (G-CEPIA1er for green, mito-RCaMP1h for red)를 통해 동시에 측정하였다. 흥미롭게도 다른 세포와 같이 신경세포의 dendrite (수상돌기)에서 mitochondria의 Ca2+ 흡수가 ER에서의 Ca2+ 방출에 의존한다는 것이 발견되었고, ER-mitochondria contact에 관여하는 PDZD8이라는 단백질도 발견되어, PDZD8 knockdown 조건에서는 이러한 Ca2+ 이동이 저해되어 있음이 관찰되었다(그림 3). 이에 더해 cytosolic Ca2+ 증가가 PDZD8 knockdown 조건에서 보여져 ER에서 방출된 Ca2+이 mitochondria로 가지 못할 경우 cytosol로 새어나감을 알 수 있다(그림 3)(18). 이 연구는 향후 dendrite에서 Ca2+ 조절 메커니즘을 이해하는데 ER-mitochondria contact이 반드시 고려되어야 함을 제시해 주고 있다.
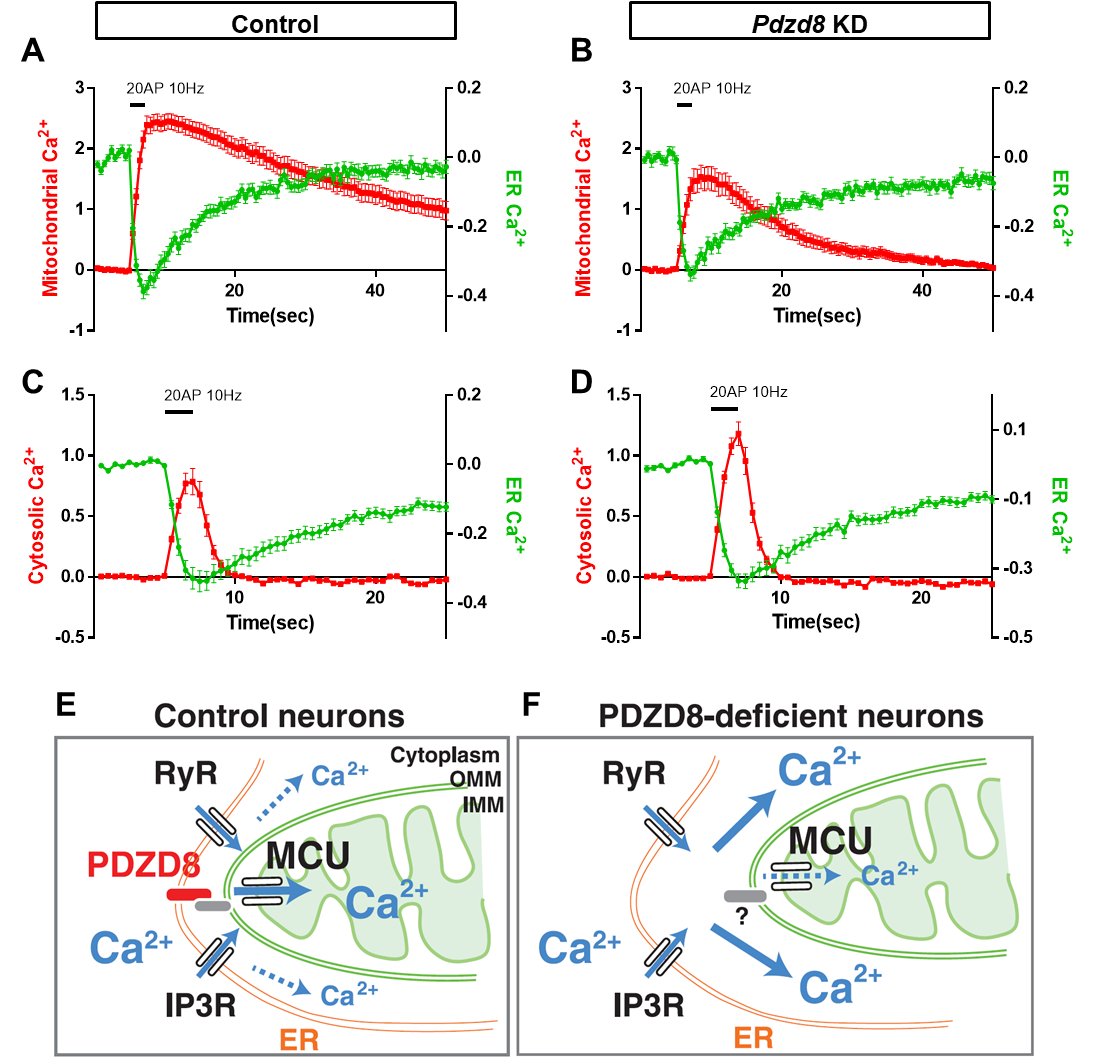
그림 3. ER-mitochondria contact을 통한 Ca2+ transfer 및 PDZD8에 의한 ER-mitochondria contact 영향.
PDZD8-deficient neuron에서 ER에서 mitochondria로 Ca2+ transfer가 저해되고, cytosolic Ca2+이 증가하게 됨.
결론 (Perspective)
위의 연구들은 다양한 이미징 방법을 통하여 기존에 관찰이 어려웠던 세포내 소기관 및 하나의 시냅스에서 칼슘 및 활성을 모니터링 하였다. 이를 통해 마이크로 스케일에서 어떠한 기능변화가 소기관의 칼슘 조절에 의해 일어날 수 있는지를 보여줬으며, 이러한 연구들은 synapse 및 신경세포의 기능을 이해하는데 기초적인 세포생물학적 지식으로 고려될 것이다. 또한 향후 신경세포 내 소기관에 의한 칼슘 조절이 시냅스 가소성 및 신경회로 기능에 어떻게 작용할지에 대한 연구가 이어질 것이다. 이는 기초 생물학적인 지식 뿐만 아니라 세포내 소기관과 관련된 퇴행성 신경질환의 병리적 특성을 밝히는데에도 핵심 요소로 적용될 것이다.
참고 문헌
1. Kornmann B and Walter P (2010) ERMES-mediated ER-mitochondria contacts: molecular hubs for the regulation of mitochondrial biology. J Cell Sci 123, 1389-1393
2. Rowland AA and Voeltz GK (2012) Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol 13, 607-625
3. Kornmann B (2013) The molecular hug between the ER and the mitochondria. Curr Opin Cell Biol 25, 443-448
4. Zhang L, Trushin S, Christensen TA et al (2016) Altered brain energetics induces mitochondrial fission arrest in Alzheimer's Disease. Sci Rep 6, 18725
5. Song W, Chen J, Petrilli A et al (2011) Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med 17, 377-382
6. Wang X, Yan MH, Fujioka H et al (2012) LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet 21, 1931-1944
7. Lewis TL, Jr., Kwon SK, Lee A, Shaw R and Polleux F (2018) MFF-dependent mitochondrial fission regulates presynaptic release and axon branching by limiting axonal mitochondria size. Nat Commun 9, 5008
8. Kwon SK, Sando R, 3rd, Lewis TL, Hirabayashi Y, Maximov A and Polleux F (2016) LKB1 Regulates Mitochondria-Dependent Presynaptic Calcium Clearance and Neurotransmitter Release Properties at Excitatory Synapses along Cortical Axons. PLoS Biol 14, e1002516
9. Rizzuto R, Pinton P, Carrington W et al (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280, 1763-1766
10. Csordas G, Thomas AP and Hajnoczky G (1999) Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J 18, 96-108
11. Suzuki J, Kanemaru K, Ishii K, Ohkura M, Okubo Y and Iino M (2014) Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat Commun 5, 4153
12. de Juan-Sanz J, Holt GT, Schreiter ER, de Juan F, Kim DS and Ryan TA (2017) Axonal Endoplasmic Reticulum Ca2+ Content Controls Release Probability in CNS Nerve Terminals. Neuron 93, 867-881 e866
13. Yeckel MF, Kapur A and Johnston D (1999) Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci 2, 625-633
14. Emptage N, Bliss TV and Fine A (1999) Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron 22, 115-124
15. Nishiyama M, Hong K, Mikoshiba K, Poo MM and Kato K (2000) Calcium stores regulate the polarity and input specificity of synaptic modification. Nature 408, 584-588
16. Finch EA and Augustine GJ (1998) Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature 396, 753-756
17. Lee KF, Soares C, Thivierge JP and Beique JC (2016) Correlated Synaptic Inputs Drive Dendritic Calcium Amplification and Cooperative Plasticity during Clustered Synapse Development. Neuron 89, 784-799
18. Hirabayashi Y, Kwon SK, Paek H et al (2017) ER-mitochondria tethering by PDZD8 regulates Ca(2+) dynamics in mammalian neurons. Science 358, 623-630
첨부파일